Automated Laboratory
APTIO Automation Line. Sample processing aligned with international clinical standards.
VIEW MORE
Unlimited Accessibility
A dedicated logistics network that supports healthcare institutions nationwide.
JOIN OUR NETWORK
Since 1948, Advancing Clinical Diagnostics
Access tomorrow’s diagnostics today. Advanced medical technology in the hands of trusted experts.

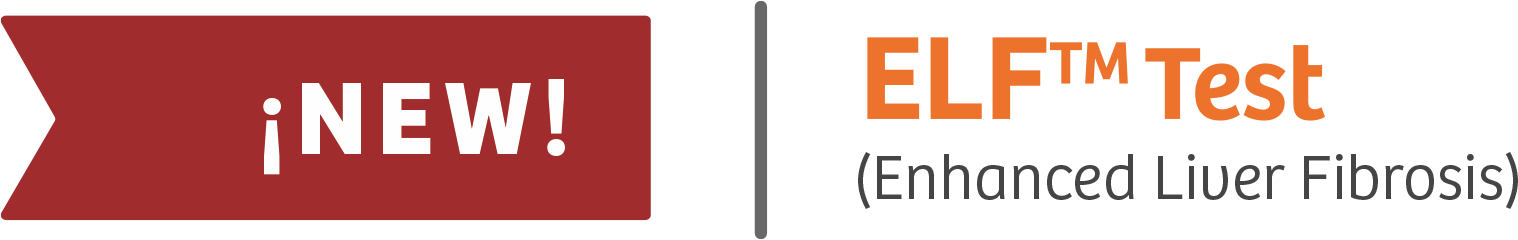
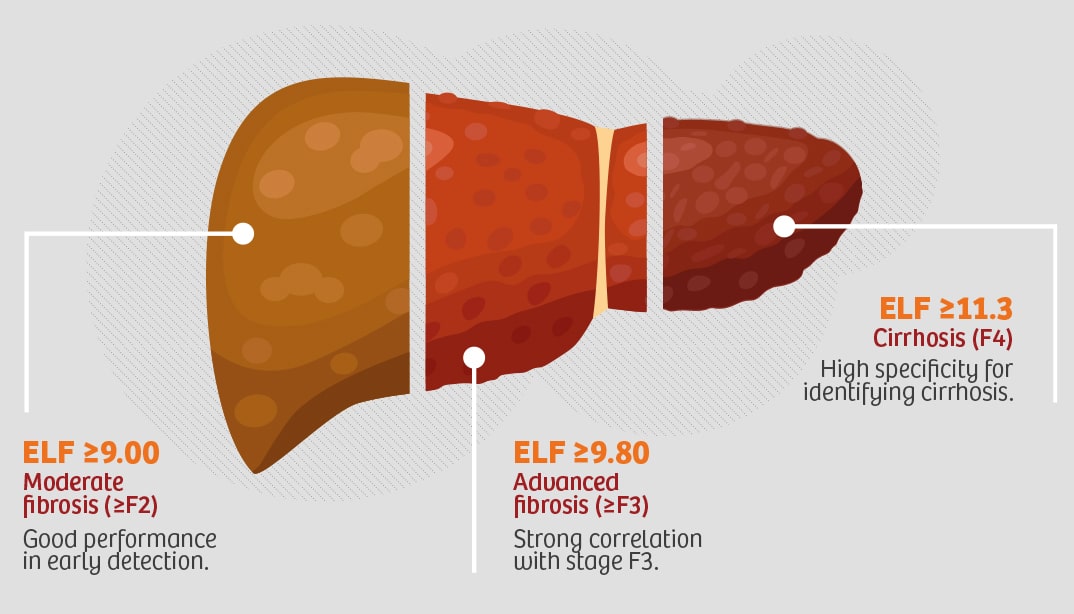
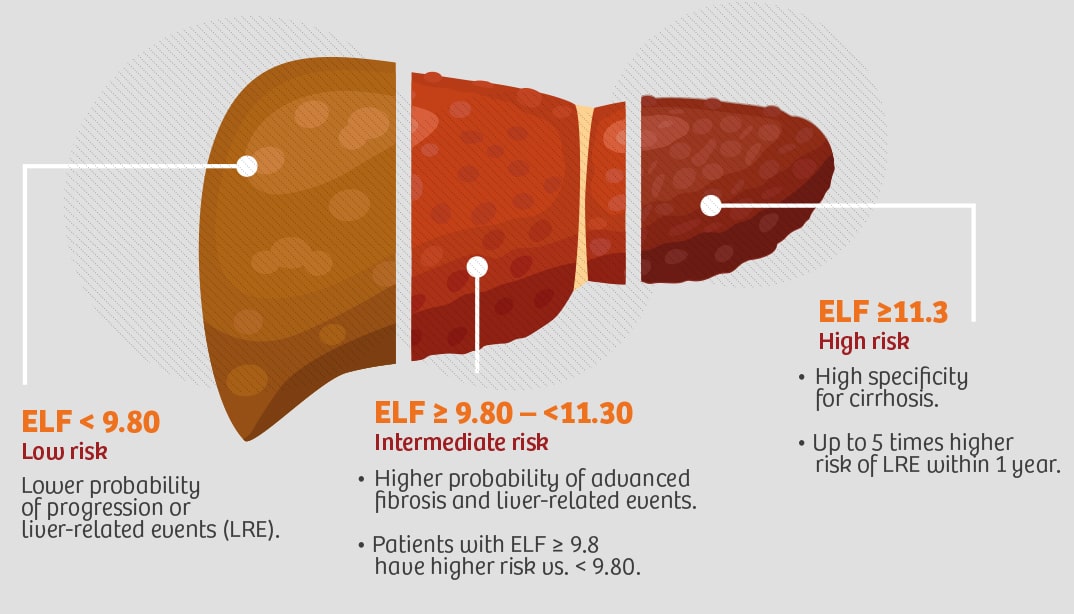
ELF™ Test (Enhanced Liver Fibrosis)
First laboratory in Argentina to offer this
innovative test.
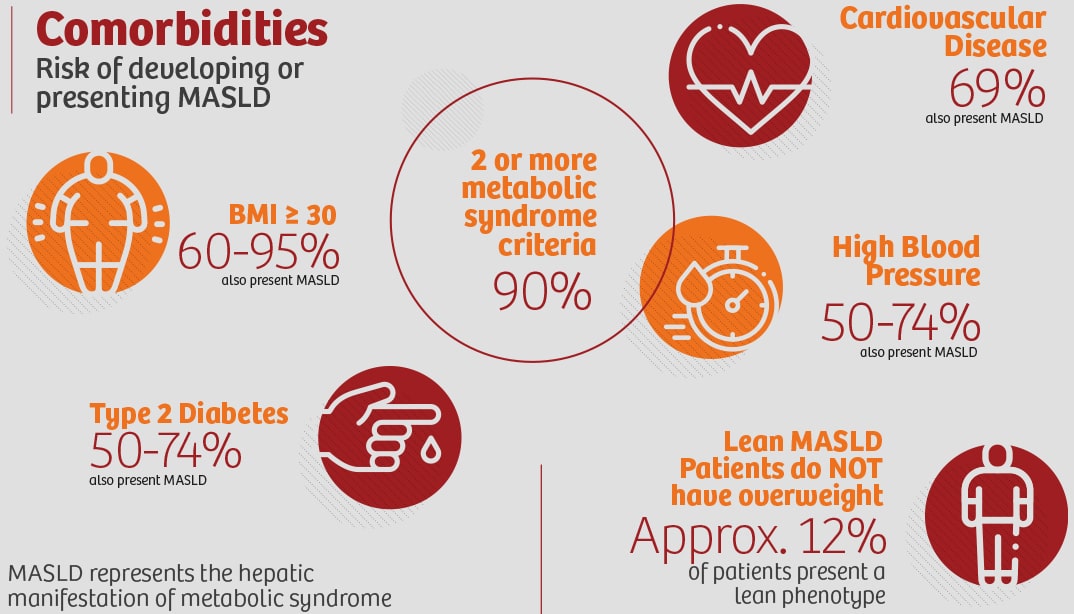
Tackling a 'not-so-silent'
public health challenge with precision.